Abstract
Background DLBCL is the most common NHL subtype, accounting for around one-third of all newly diagnosed cases. Over 50% of patients with advanced-stage de novo DLBCL are cured with R-CHOP; however, 20-50% of patients will become R/R following treatment. Historically, outcomes for these patients were poor; in a meta-analysis, the ORR to the next line of therapy in patients with R/R DLBCL was 26% (Crump, et al. Blood. 2017;130:1800-8). Recently approved therapies for R/R DLBCL in the ≥3L setting, including CAR-T cell therapies, have significantly improved outcomes for patients. However, many patients remain unsuitable candidates for CAR-T cell therapy upon relapse and thus a high unmet need for novel R/R DLBCL treatments remains.
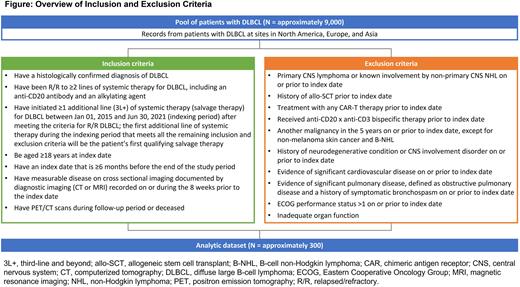
Large-scale, patient-level, real-world (RW) evidence studies can provide a better understanding of outcomes with currently available therapies in routine clinical practice and contextualize results from uncontrolled clinical trials. In the ORCHID study, we aim to identify a large cohort of approximately 700 patients with R/R DLBCL initiating ≥3L systemic therapy from over 30 clinical sites in 13 countries across North America, Europe, and Asia to characterize RW treatment patterns and outcomes. Among them, we will create an analytic cohort with approximately 300 patients who have measurable disease at baseline and PET/CT scans during follow-up, unless they died.
Study Design and Methods ORCHID (NCT05338892) is an electronic medical record-based observational, retrospective cohort study evaluating treatment patterns and outcomes in patients with R/R DLBCL who initiate currently available systemic therapies after at least 2L of systemic therapies, including an anti-CD20 antibody and an alkylator. The study population will be selected based on similar eligibility criteria as the R/R DLBCL arm of the odronextamab ELM-2 trial (NCT03888105) (Figure). Patients must have measurable disease and must have initiated at least 1 additional line of systemic therapy (salvage therapy) for DLBCL between Jan 01, 2015 and Jun 30, 2021 (indexing period) after meeting the criteria for R/R DLBCL.
ORCHID is designed to contextualize outcomes from patients with R/R DLBCL enrolled in contemporaneous single-arm studies. To estimate RW ORR by independent central review (ICR), analytic cohorts will be created from the ORCHID population based on data requirements and matching feasibility. Patients eligible for inclusion in ORCHID will be propensity score matched and/or weighted to each patient in the ELM-2 trial (anticipated cohort, n=127). To identify variables for inclusion in the propensity score model, a systematic literature review (Prognostic Factors and Effect Modifiers in Patients with Relapsed or Refractory DLBCL Who Failed at Least 2 Lines of Therapy; registered on PROSPERO as CRD42022307557) was conducted. Three key opinion leaders categorized prognostic variables as being of "very high importance", "high importance", "mild importance", "low importance", and "not important” and ranked them for inclusion in the propensity score model. The most important prognostic variables identified include early chemoimmunotherapy failure, Eastern Cooperative Oncology Group performance status, refractory to last line of therapy, number of prior treatment lines, double or triple-hit lymphoma, age, International Prognostic Index, Ann Arbor stage, and lactate dehydrogenase.
The primary endpoint of ORCHID is ORR according to the Lugano classification of malignant lymphoma and as assessed by ICR. Secondary outcomes include ORR according to the Lugano classification and as assessed by the treating physician, as well as complete response rate, progression-free survival, duration of response, and disease control rate by both the treating physician and ICR assessments. Overall survival and time to next treatment will also be evaluated. Subgroups of interest will include historical (Jan 01, 2015 through Nov 12, 2019) versus contemporary treatment (Nov 13, 2019 through Jun 30, 2021), CAR-T cell therapy versus other salvage regimens, and geographic region. Sensitivity analyses will be conducted in expanded cohorts to assess the impact of eligibility criteria and availability of scans.
When complete, ORCHID will provide important information on the RW outcomes of patients with DLBCL with high unmet need and provide a valuable source of control data for comparative studies in R/R DLBCL.
Disclosures
Thieblemont:AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel Support; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel Support, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Other: Travel Support; Bristol Myers Squibb: Consultancy, Honoraria, Other: Travel Support. Ma:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hampp:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Harnett:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; Pfizer Inc.: Current equity holder in publicly-traded company. Sobel:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; Pfizer: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Jalbert:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Quek:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Wei:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wu:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Mastey:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wang:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Bajwa:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Maissenhaelter:IQVIA: Current Employment; Regeneron Pharmaceuticals, Inc.: Consultancy. Aggarwal:IQVIA: Current Employment; Amgen: Ended employment in the past 24 months. Chaudhry:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Mohamed:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Ambati:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Bachy:Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Amgen, BMS: Research Funding; Hospices Civils de Lyon: Current Employment. Damaj:Takeda: Consultancy, Other: Travel, accommodation, expenses; Blueprint Medicines Corporation: Consultancy; Thermofisher: Consultancy; Takeda: Other: Travel, accommodation, expenses; Pfizer: Other: Travel, accommodation, expenses; AbbVie: Other: Travel, accommodation, expenses.
OffLabel Disclosure:
Off-Label Drug Purpose: The abstract describes the study design and methods of an observational, retrospective cohort study of real-world treatment patterns and outcomes in adults with R/R DLBCL (ORCHID), which aims to contextualize outcomes in contemporaneous single-arm studies such as the ELM-2 trial of odronextamab.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal